
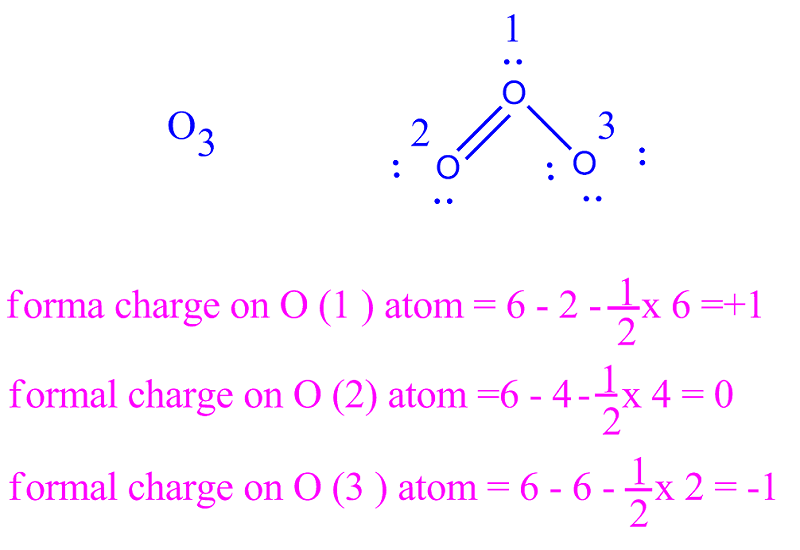
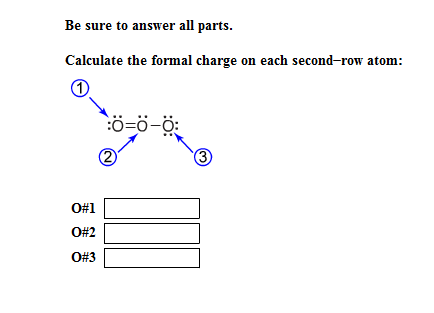
Formal charges are drawn in close proximity to the atom bearing the charge. In contrast, this convention is not followed in inorganic chemistry. In organic chemistry convention, formal charges are an essential feature of a correctly rendered Lewis–Kekulé structure, and a structure omitting nonzero formal charges is considered incorrect, or at least, incomplete. The formal charge system is just a method to keep track of all of the valence electrons that each atom brings with it when the molecule is formed. It is important to keep in mind that formal charges are just that – formal, in the sense that this system is a formalism. Draw a circle around the atom for which the formal charge is requested (as with carbon dioxide, below).

Carbon double bonded to both oxygen atoms (carbon = 0, oxygens = 0, total formal charge = 0)Įven though all three structures gave us a total charge of zero, the final structure is the superior one because there are no charges in the molecule at all.Carbon single bonded to one oxygen and double bonded to another (carbon = +1, oxygen double = 0, oxygen single = −1, total formal charge = 0).Carbon single bonded to both oxygen atoms (carbon = +2, oxygens = −1 each, total formal charge = 0).There are different ways to draw the Lewis structure Example: CO 2 is a neutral molecule with 16 total valence electrons.It can also be found visually as shown below. Where V is the number of valence electrons of the neutral atom in isolation (in its ground state) L is the number of non-bonding valence electrons assigned to this atom in the Lewis structure of the molecule and B is the total number of electrons shared in bonds with other atoms in the molecule.


 0 kommentar(er)
0 kommentar(er)
